Microdroplet Chemistry for the Synthesis of Gold Nanostructures
Jae Kyoo Lee
Chemical reactions in confined environments behave differently than the same ones in bulk solution. We have found that chemical reactions are significantly accelerated in micron-sized aqueous droplets (microdroplets) by a factor over 105 for various reactions including protein unfolding, protein-ligand binding, chlorophyll demetallation, and hydrogen-deuterium exchange (PNAS 2015, Q Rev Biophys 2016 and 2017). We also found that the reactions with a high thermodynamic barrier can spontaneously occur in microdroplets by lowering the entropy change at the water surface (PNAS, 2017). The synthesis of gold nanostructures has received widespread attention owing to many important applications. We report the accelerated synthesis of nanoparticles (AuNPs) as well as the reducing-agent-free and template-free synthesis of nanowires in aerosol microdroplets. At first, the AuNP synthesis was carried out by fusing two aqueous microdroplet streams containing chloroauric acid and sodium borohydride. AuNPs (~7 nm in diameter) were produced within 60 µs at the rate of 0.24 nm/µs. Compared to bulk solution, microdroplets enhanced the size and growth rate of AuNPs by factors of about 2.1 and 1.2 x 105, respectively.
Later, we found that gold nanoparticles and nanowires (~7 nm wide, and >2000 nm long) were also formed in microdroplets in the absence of any added reducing agent, template, or externally applied charge. Thus, water microdroplets not only accelerate synthesis of AuNPs by orders of magnitude but they also cause spontaneous formation of gold nanostructures. We argue that this spontaneous formation of the nanostructures is due to the reduction capability of water microdroplets with the aid of electric field at or near water-air interface.
We anticipate various application of the new method of synthesizing metal nanostructures in microdroplets without using reducing agents or templates for catalysis, solar energy conversion, medical imaging, tumor treatment, and flexible display.
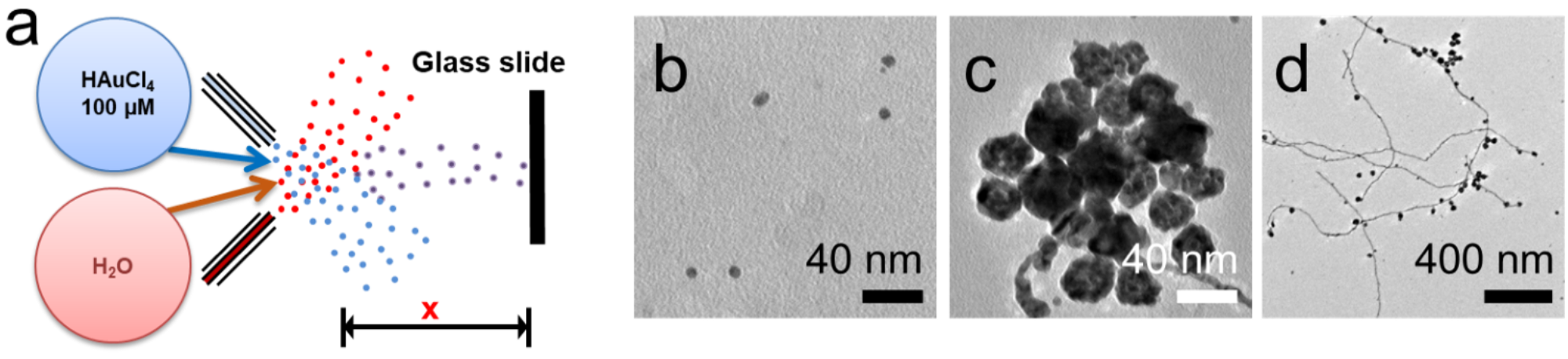
Figure 1: (a) Set up for the synthesis of gold nanostructures in aqueous microdroplets. TEM images of (b) gold nanoparticles, (c) gold nanoparticle aggregates, and (d) gold nanowires accompanying with AuNP and AuNP aggregates
1. Jae Kyoo Lee, Devleena Samanta, Hong Gil Nam, Richard N. Zare, Nature Communications, under revision. 2. Inho Nam, Jae Kyoo Lee, Hong Gil Nam, and Richard N. Zare, Proc Natl Acad Sci U S A 114, 12396 (2017) 3. Jae Kyoo Lee, Hong Gil Nam, and Richard N. Zare, Quarterly Reviews of Biophysics 50 e2 (2017) 4. Jae Kyoo Lee*, Shibdas Banerjee*, Hong Gil Nam, Richard N. Zare, Quarterly Reviews of Biophysics 48(4), 437 (2015). 5. Jae Kyoo Lee, Samuel Kim, Hong Gil Nam, Richard N. Zare, Proc Natl Acad Sci U S A 112(13), 3898 (2015).
