Mechanistic Study of Reaction Acceleration and Heterogeneous Catalytic Oil Upgrading in Microdroplets
Yin-Hung Lai, Maria T. Dulay
Although it has been reported that microdroplets, typically generated by electrospray, provide unique environments for accelerating a variety of chemical reactions, the mechanisms underlying this phenomenon are not fully understood. Reaction kinetics in microdroplets are often found to be significantly faster than the corresponding bulk-phase kinetics, suggesting the important role of interfacial chemistry. The rate acceleration in a model system, the nucleophilic opening of limonene oxide with morpholine, has been investigated.[1] By utilizing micro-particle imaging velocimetry (micro-PIV) to measure sizes and velocities of microdroplets,[2] significant factors contributing the rate acceleration including the surface area to volume ratio and the reaction time have been evaluated. Decreasing the size of microdroplets and increasing the reaction time dramatically increase conversion to product. It is our hope that a fundamental understanding of the mechanisms of droplet acceleration will help in future preparative-scale chemical synthesis.
Caused by the continuous depletion of conventional oil reserves, to meet growing demand for fuels, development of alternative fuels by oil upgrading, that is, to break down asphaltene molecules into smaller fragments, becomes essential. We report a novel ambient method that expedites the degradation rate of asphaltenes by spraying sample microdroplets onto a charged paper surface containing catalytic TiO2 nanoparticles.[3] In our method, an additional supply of hydrogen source is not needed. Unlike conventional photocatalysis, degradation that takes place at the interface between oil droplets and the charged surface is electric field induced and accomplished on the sub-millisecond time scale. Moreover, the fragmentation yield is further increased by recycling the process, that is, by retreating the oil sample that has contacted the solid support. We have shown a proof-of-concept process demonstrating the effectiveness of this technique for model compounds (rubrene and hematoporphyrin) as well as undiluted/diluted crude oil samples. Recently, we have found a way to degrade compact asphaltenes and are optimizing the capability of the method.
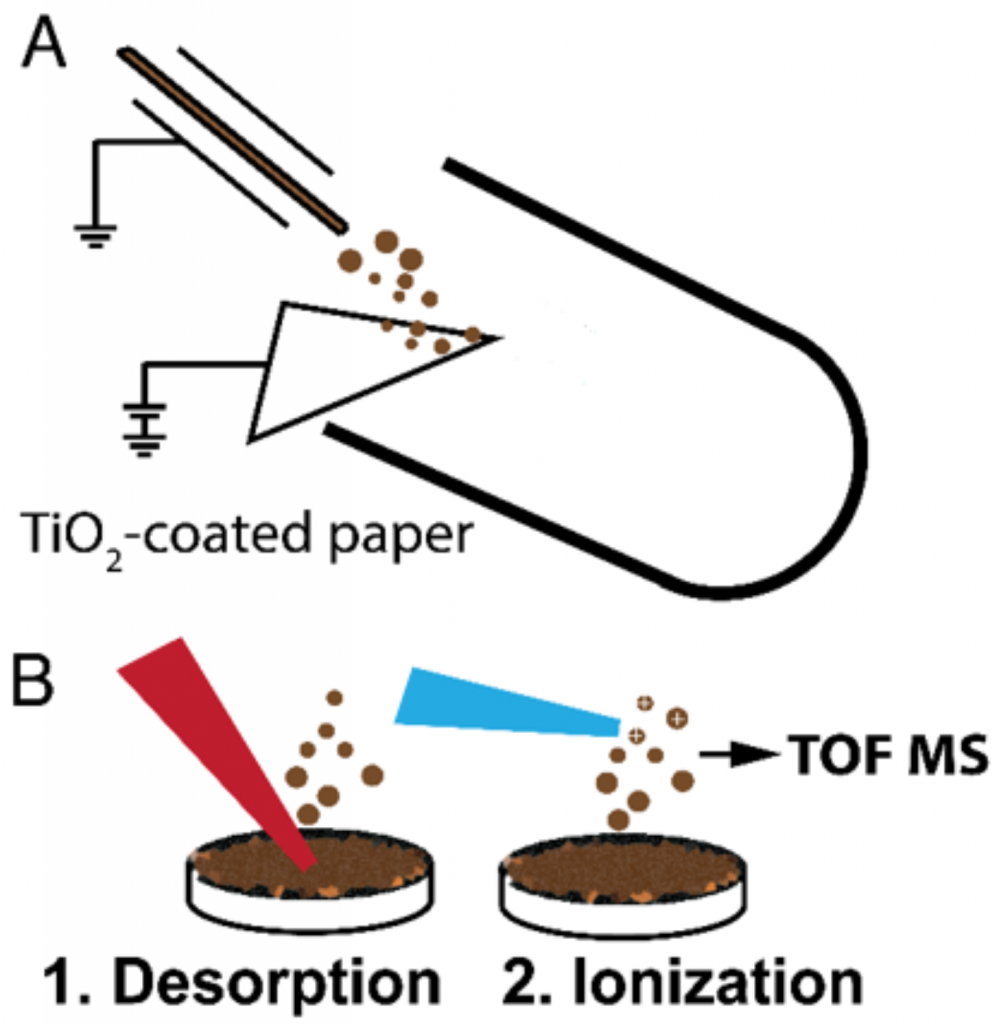 Workflow for the heterogeneous catalytic degradation of asphaltenes
Workflow for the heterogeneous catalytic degradation of asphaltenes
(A) a spray source is used to accommodate sample solution (organic solution, in brown color), which sprays sample microdroplets onto the aqueous wet surface of the filter paper containing anatase TiO2 nanoparticles. The spray source of sample solution is electrically grounded. High voltage is applied to the wet paper coated with TiO2 nanoparticles; (B) Extracted sample is deposited on a glass platter and analyzed using laboratory-built laser desorption laser ionization mass spectrometry (L2MS).
[1] Y.-H. Lai, S. Sathyamoorthi, R. M. Bain, R. N. Zare, J. Am. Soc. Mass Spectrom. 2018. [2] E. T. Jansson, Y.-H. Lai, J. G. Santiago, R. N. Zare, J. Am. Chem. Soc. 2017, 139, 6851-6854. [3] Y.-H. Lai, Z. Zhou, B. Chanbasha, R. N. Zare, Energy Fuels 2017, 31, 12685–12690.
