In-Situ Ambient Ionization Mass Spectrometry for Mechanistic Studies of Organometallic Catalysis
Katherine Walker
High-resolution electrospray ionization mass spectrometry (ESI-MS) is an powerful technique for identifying organometallic intermediates in catalytic reactions.1-3 While identifying intermediates is not sufficient for fully understanding a mechanism, it provides one important piece of information that can be combined with traditional kinetic and isotope labeling studies to provide insights on catalytic mechanisms. Additionally, in-situ techniques such as pressurized sample infusion (PSI, Figure 1) can be used to continuously monitor the speciation of organometallic complexes.4 Comparing a compound’s speciation over time (by MS) to the consumption of starting material and formation of products (by NMR or other offline techniques) provides powerful information about that compound’s role in the catalytic cycle. Much of this research is highly collaborative with existing collaborations between the Waymouth Lab and Du Bois Lab (Stanford), and the Muldoon Lab (Queen’s University, Belfast).
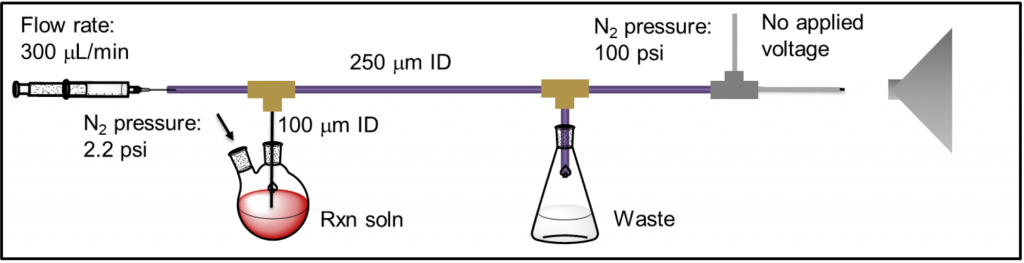
Figure 1: Example setup for pressurized sample infusion
The Pd complex [(PBO)Pd][OTf]2 is a selective cationic catalyst for the oxidation of styrene and styrene derivatives to their corresponding ketones using H2O2 as the terminal oxidant.5 Using ESI-MS, two key species: LPdOOH+, and LPdCH2COPh+ were discerned (Figure 2).6 Then, through a series of kinetic and isotope labeling studies, it was shown that the unexpected latter species was indeed an on-path intermediate. Furthermore, this “palladium enolate” has been implicated as an off-path species in other common palladium-catalyzed alkene oxidations with peroxides, such as Sigman’s “Quinox” and tbutyl hydroperoxide system.7 A final Pd alkylhydroperoxide intermediate (LPdCH2C(OOH)Ph) was identified when an H-atom donor was added (Figure 2).
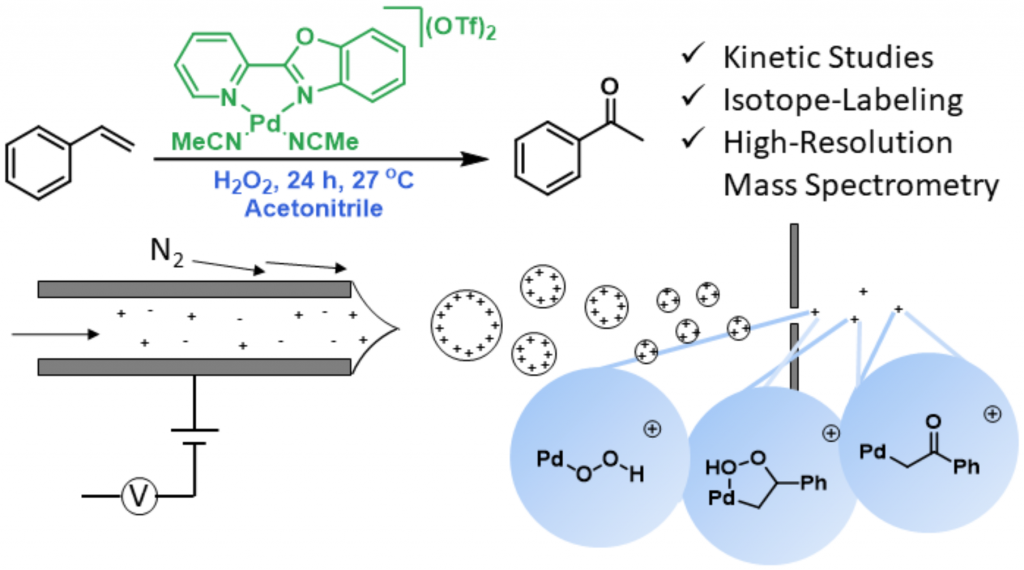
Figure 2: Styrene oxidation by a PBOPd(OTf)2 catalyst and H2O2 revealed key intermediates
The Ru complex (DTBPY)2RuCl2 has been shown to be a competent C-H hydroxylation catalyst for substrates that contain amines and azacycles under acidic and strongly oxidizing conditions.8 Recently, with PSI-MS, the speciation of the Ru(II) catalyst during a reaction was monitored, and Ru(IV), Ru(V), and Ru(VI) were all observed (Figure 3). Both Ru(V) and Ru(VI) may be responsible for product formation. Also, the loss of ligand and the formation of oxidized ligand occurs concomitantly with the production of high oxidation Ru. Ongoing studies are being conducted to quantify the ligand loss and the other degradation pathways.
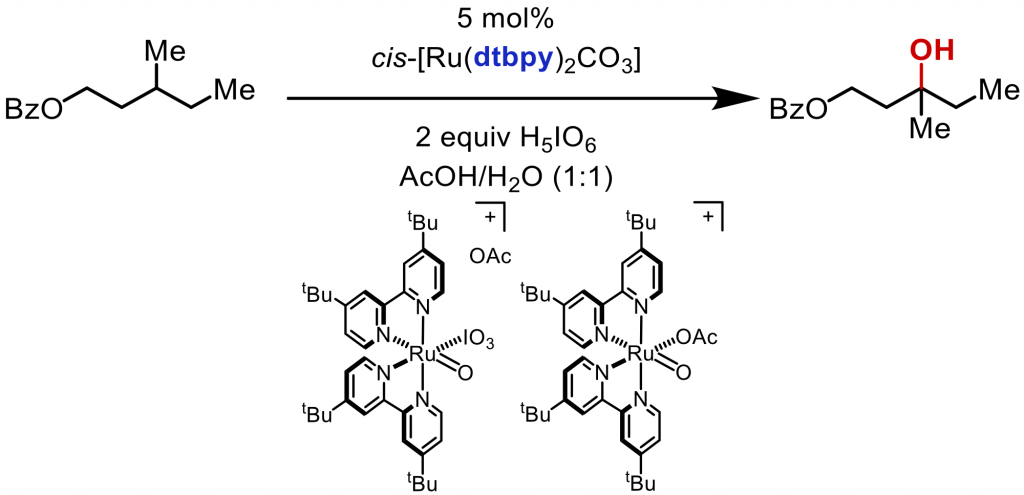
Figure 3: Ru-catalyzed C-H hydroxylation
1. Ingram, A. J.; Boeser, C. L.; Zare, R. N., Chemical Science 2016, 7, 39-55.
2. Davis, D. C.; Walker, K. L.; Hu, C.; Zare, R. N.; Waymouth, R. M.; Dai, M., J. Am. Chem. Soc. 2016, 138, 10693-10699.
3. Ingram, A. J.; Walker, K. L.; Zare, R. N.; Waymouth, R. M., J. Am. Chem. Soc. 2015, 137, 13632-13646.
4. Theron, R.; Wu, Y.; Yunker, L. P. E.; Hesketh, A. V.; Pernik, I.; Weller, A. S.; McIndoe, J. S., ACS Catalysis 2016, 6, 6911-6917.
5. Cao, Q.; Bailie, D. S.; Fu, R.; Muldoon, M. J., Green Chemistry 2015, 17, 2750-2757.
6. Walker, K. L.; Dornan, L. M.; Zare, R. N.; Waymouth, R. M.; Muldoon, M. J., J. Am. Chem. Soc. 2017, 139, 1249512503.
7. Michel, B. W.; Steffens, L. D.; Sigman, M. S., J. Am. Chem. Soc. 2011, 133, 8317-8325.
8. Mack, J. B. C.; Gipson, J. D.; Du Bois, J.; Sigman, M. S., J. Am. Chem. Soc. 2017, 139, 9503-9506.
