Design of Nanoparticles for Biomedical Applications
Katy Margulis
We design organic nanoparticles for efficient delivery of a wide spectrum of therapeutic agents. One specific interest is to develop albumin nanoparticles that modulate the immune system to help eradicate cancers. We conjugate specific targeting agents, such as growth factors, to the surface of the particles in order to selectively deliver immunomodulating drugs to the cells of interest (Figure 1). The bioavailability, potency, and target-specificity of the resultant nanoparticles are tested in vivo in the Smith lab, Stanford Medical School. During the in vivo studies, endogenous chemical changes caused by the drug and/or the nanoparticles are also monitored by the desorption electrospray ionization mass spectrometry imaging (DESIMSI). We have two objectives: first, to maximize the specificity of the nanoparticle uptake to myeloid immune cells and second, to measure how the nanoparticles change the immune system around growing tumors and the effect this has on treatment effectiveness.
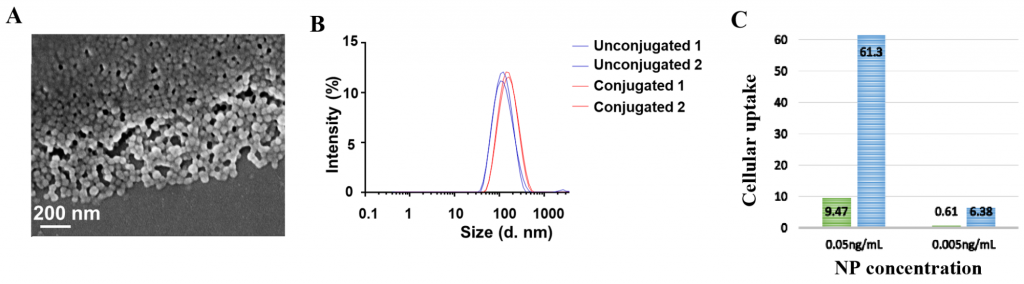
Figure 1. (A) Scanning electron microscopy image of albumin nanoparticles; (B) Particle size increase of albumin nanoparticles following conjugation to growth factor; (C) Increase in uptake of conjugated nanoparticles (blue) vs. unconjugated (green) by immune cells.
Our other interest involves template-free fabrication of non-spherical polymer nanoparticles, which might have unique impacts in vivo.1 In collaboration with the Waymouth lab (Stanford Chemistry), we recently reported an operationally simple method to form polymeric cubic nanoparticles by the aqueous self-assembly of amphiphilic triblock copolymers containing central poly (ethylene oxide) block and terminal poly(trimethylene carbonate/ dithiolane) blocks,2 followed by evaporationinduced crystallization of the central blocks (Figure 2). The versatile chemistry of the hydrophobic dithiolane cores of these micelles provides a facile mechanism for chemical or photochemical crosslinking to fix the hierarchical structure following self-assembly.3
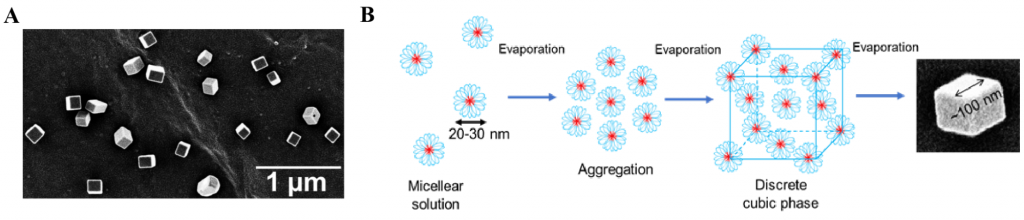
Figure 2. (A) Scanning electron microscopy image of nanocubes formed on aluminum stub; (B) Possible mechanism of cube formation through micelle aggregation into nanometric cubic phase.
1. J. F. Alexander, V. Kozlovskaya, J. Chen, T. Kuncewicz, E. Kharlampieva, B. Godin, Adv. Healthcare Mater. 2015, 4, 2657-2666.
2. G. A. Barcan, X. Y. Zhang, R.M. Waymouth, JACS 2015, 137, 5650-5653.
3. K. Margulis, X. Y. Zhang, L.M. Joubert, C. J. Tassone, K. Bruening, R.N. Zare, R.M. Waymouth, Angew. Chem. Int. Ed. 2017, 129, 16575- 16580.
