Abiotic Production of Sugar Phosphates and Ribonucleosides in Aqueous Microdroplets
Jae Kyoo Lee, Jooyoun Kang
Phosphorylation is an essential chemical reaction for life. This reaction generates fundamental cell components, including building blocks for RNA and DNA, phospholipids for cell walls, and adenosine triphosphate (ATP) for energy storage. We find that the phosphorylation of various sugars to form sugar-1-phosphates can proceed spontaneously in aqueous microdroplets containing a simple mixture of sugars and phosphoric acid (Figure 1A).1 The yield for D-ribose-1phosphate reached over 6% at room temperature, giving a ΔG value of –1.1 kcal/mol, much lower than +5.4 kcal/mol for the reaction in bulk solution. The temperature dependence of the product yield for the phosphorylation in microdroplets revealed a negative enthalpy change (ΔH = –0.9 kcal/mol) and a negligible change of entropy (ΔS = 0.0007 kcal/mol·K). Thus, the spontaneous phosphorylation reaction in microdroplets occurred by overcoming the entropic hurdle of the reaction encountered in bulk solution. Moreover, ribonucleosides are generated in aqueous microdroplets containing D-ribose, phosphoric acid, and four types of nucleobases (Figure 1B).2 Aqueous microdroplets containing 15 mM D-ribose, 15 mM phosphoric acid, and 5 mM of the nucleobase, uracil, adenine, cytosine or hypoxanthine are electrosprayed from a capillary at +5 kV into a mass spectrometer at room temperature and one atmosphere pressure with 3 mM divalent magnesium ion (Mg2+) as a catalyst. Mass spectra show the formation of ribonucleosides during the flight time of about 50 µs with a yield of 2.5% of uridine (U), 2.5% of adenosine (A), 0.7% of cytidine (C), and 1.7% of inosine (I). In the case of uridine, no catalyst is required. Guanine failed to form guanosine (G) under the same conditions, owing to the insolubility of guanine in water. However, inosine can be substituted for guanosine and makes a base pair with cytidine. Thus, a full set of ribonucleosides to generate the purinepyrimidine base pairs A-U and I-C are spontaneously generated in aqueous microdroplets under similar mild conditions. The large amount of aqueous interface of microdroplets possesses a strong electric field, which influences the organization of molecules, and the kinetics and thermodynamics of biological reactions. Therefore, the reaction acceleration or the thermodynamic improvement in biological reactions illustrates that metabolism of biomolecules in a confined environment, like that of a cell, could behave differently than what has been reported through assays using bulk solutions.
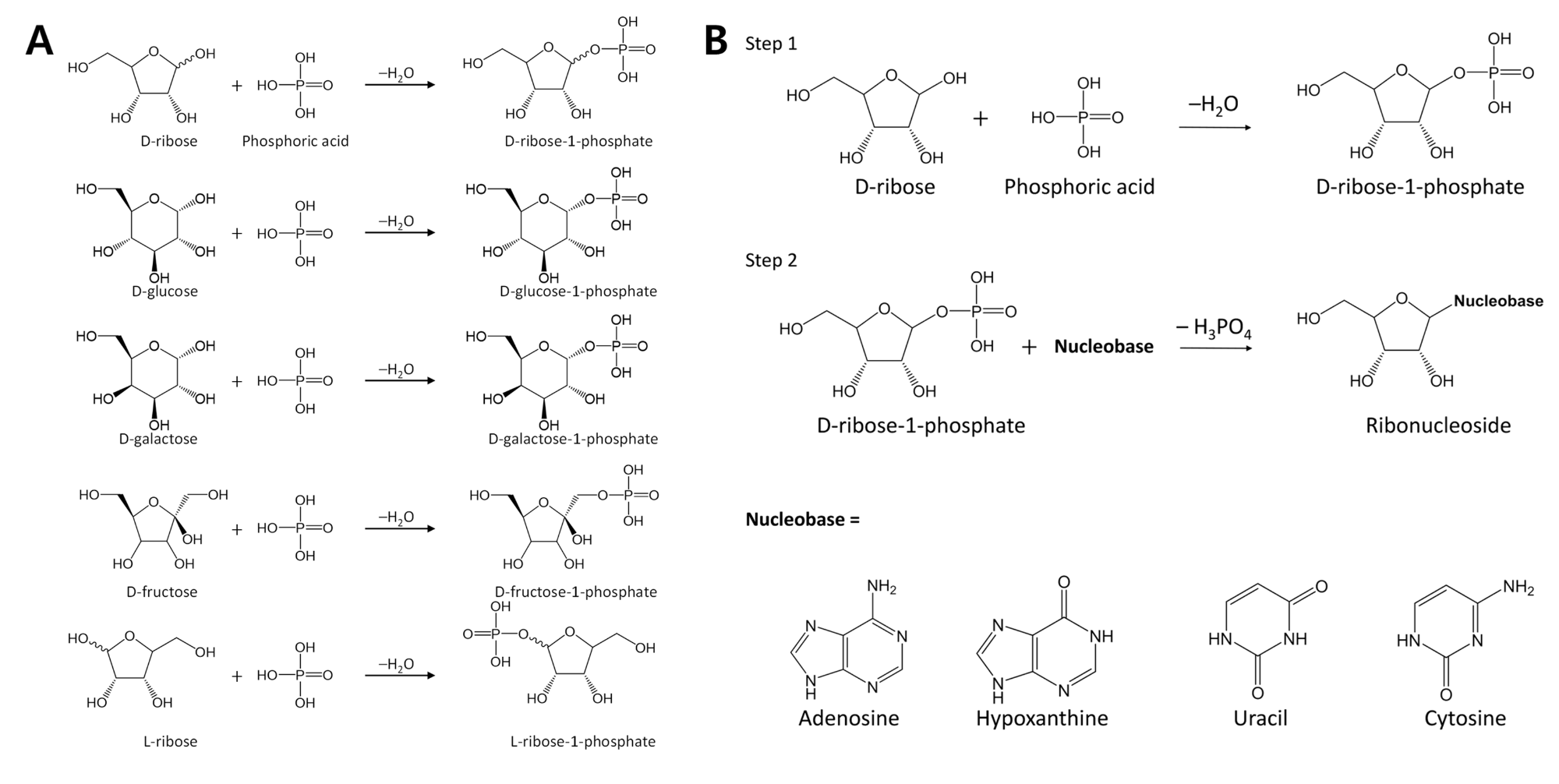
Figure 1: Phosphorylations and ribosilatons proceed spontanueously and rapidly (in 100 µs or less) in aqueous microdroplets.
1. I. Nam, J. K. Lee, H. G. Nam, R. N. Zare, Proc. Nat. Acad. Sci. (USA) 114, 12396 (2017). 2. I. Nam, H. G. Nam, R. N. Zare, Proc. Nat. Acad. Sci. (USA) 115, 36 (2018).
