Do bubbles in Guinness go down?
We are used to seeing bubbles float up in a glass of beer, but bubbles in Guinness appear to break the rules.
Do the bubbles really sink in Guinness, or is it just an illusion?
If the bubbles go down, where do they go? Why do they all end up at the head?
Here we show you some video proof that the bubbles in Guinness do actually go down the inside of the glass just after the beer is poured. To learn more, click on to one of the bubbles opposite, or use one of the links below.
Why do the bubbles go down?
 |
Let’s start at the point where you have just poured your pint of Guinness, and it is starting to settle. At the inside surface of the glass, the bubbles are touching the walls of the glass and they experience drag – just in the same way as you can feel if you slide your finger along a glass surface. At the center of the glass, the bubbles are not touching the walls, and are free to go up: this is what bubbles of gas really want to do when they are in a liquid, as we are used to seeing. |
 |
The bubbles at the center rise rapidly until they get to the top, just below the head (the “froth”). In doing this, they have pushed and pulled the surrounding liquid with them. At the top, this liquid flowing upwards hits the surface and flows outwards towards the edges of the glass. |
 |
The current is directed downwards by the edges of the glass. As the flow moves downwards in waves, it pushes and pulls the bubbles that are hanging around at the edges of the glass. The flow can be seen as the dark lines of liquid (no bubbles) that wave quickly down the inside of the glass. |
 |
What goes around comes around. More bubbles flow up at the center, and the circulation continues. |
 |
Eventually the settling process comes to an end. More and more bubbles have been deposited into the head of the beer during the settling, and the cycle loses momentum. |
 |
In summary: bubbles at the center rise up and create a circulation in the glass. The circulation causes bubbles at the edge of the glass to be pushed downwards. |
Does it only happen in Guinness?
 |
No, it can happen in any liquid. There are a number of things about Guinness that make it easier to see though. Firstly, the bubbles produced are small, making them more easily pushed around by flowing liquid. The bubbles are small because they have been released at high pressure through fine holes (when poured from the tap or from the “widget“). The gas in the bubbles is also important. The fizz in most lager-beers (and soda) is carbon dioxide, which is more easily dissolved into the liquid. This is why you can see streamers of bubbles appear to form out of nowhere at the side of a glass of beer or cola: the dissolved carbon dioxide undissolves to form bubbles at tiny defects in the glass surface: they continue to grow, ingesting more dissolved carbon dioxide as they go up (as shown, left, for cola). In Guinness, the gas is nitrogen (which makes up roughly three quarters of the air we breathe). Nitrogen does not dissolve as well in liquid as carbon dioxide, and so the bubbles do not grow like in lager or cola. Another factor is the contrast in the colours of the very dark liquid and the light cream bubbles in Guinness, making the waves and bubbles more obvious. |
 |
To prove downflow of bubbles happens in other liquids, we looked at other draught-flow beers (e.g., Boddingtons). The results are exactly the same as with Guinness. In this clip (Boddingtons) you see a similar downflow of the bubbles. A bubble-free wave of liquid can be seen very clearly. |
 |
Not being content with beer, we set out to make bubbles go down in other liquids. To do this we devised a “bubble-maker”, as shown in the picture opposite. This is just a piece of tube with fine holes in the end (at the bottom) through which we can blow gas to make small bubbles. The liquid in the glass is just plain water. The bubbles go up the center of the glass, just like in the Guinness, and create a circular flow that causes bubbles at the inside edge near the top to be pushed downwards. You can see the result in the next clip below. |
 |
In this clip you can see the bubbles produced at the top of the glass of water by the bubble-maker. The bubbles are much larger than are produced in Guinness. This is because they have not been produced at high pressure as they have in Guinness, a process known as “gas breakout”. |
 |
Another way of making bubbles go down is to simply add a fizzing tablet to a glass of water. These are not so easily seen with the eye, but easily seen with the fast camera: as shown in the clip here. Another way of visualizing the flow is to use a suspension of powder in liquid giving what is known as a rheoscopic fluid. The particles of the additive powder allow the fluid flows to be visualised. Bottles of rheoscopic fluids can be purchased at good science or teacher supply stores. |
Why is this effect important?
The effects are important because they tell us about how liquids flow, for example, in industrial processes and manufacture. Liquid flows are important in a wide range of circumstances ranging from medicine to oceanography. Drinks and food manufacturers also spend a considerable amount of effort and money to make their products appealing: easy pouring and a nice head is one of the aspects that we consumers look for in our beer.
About the filming
Our initial attempts to video the bubbles in Guinness with a regular video camera were all in vain. The bubbles are just too small, and too fast to see clearly. We borrowed a high-speed digital camera (Kodak HS4540), which is capable of taking up to 4,500 full frames in one second. Below is a picture of the setup. The camera takes the pictures and passes the images to the electronics box where they are stored. These can then be recorded to VCR, or transferred as digital images to the computer.

How small? How fast are they?
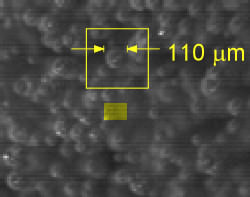 Most of the footage you can see on this site was taken at 750 frames per second, and is replayed at 25 frames per second – effectively slowing the bubbles down 30 times.
Most of the footage you can see on this site was taken at 750 frames per second, and is replayed at 25 frames per second – effectively slowing the bubbles down 30 times.
We used a zoom lens at around 6 times magnification to see the bubbles, giving a field of view (size of the whole picture) of about 3 mm or 1/8″ – about the same as the width of two pennies.
What can we say about the size of the bubbles? We can measure them from the individual frames: see the picture opposite. We find that they are about 40 to 120 micrometers (1 to 4 thousandths of an inch) in diameter. That makes them roughly the size of human hairs (a human hair is roughly 100 micrometers wide).
And how fast are they going? The time between the two frames below is 20 thousandths of a second. The same single bubble has been circled in yellow at both times.
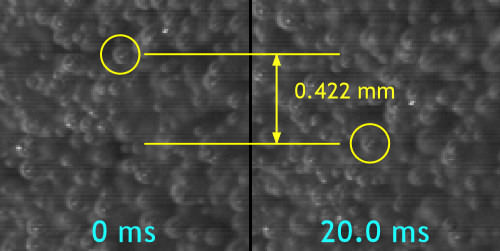
So the bubble travels 0.422 mm (0.0166″) in 0.02 s. At that speed it could travel 2.11 cm (0.831″) in 1 second, about 0.076 km (0.047 miles) per hour, or about a mile a day!
Hold on a second! You said they were fast – right? 0.047 miles per hour doesn’t sound fast to me!
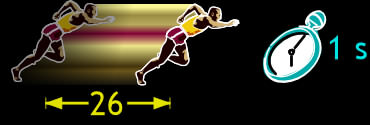
Yes, that’s true. But we are mostly used to hearing about speeds of things that are much bigger – like cars and planes. To put it into perspective, let’s see how our bubble stacks up against a 100 m athelete. How wide (from front to back) is a 100 m runner? Let’s say 40 cm (16″). Our bubble is only 0.011 cm wide (0.0043″) – four thousand times smaller.
Our athelete does 100 m in 9.78 s, that is, 10.2 m in 1 s. Compared to his width, that’s 26 body widths in 1 second. By comparison, our bubble only does 2.11 cm in 1 second. But that’s 192 times its own diameter in 1 second – more than 7 times better than our 100 m champion!

Acknowledgements
We thank the EPSRC Engineering Instrument Pool for the loan of the Kodak hi-speed digital camera equipment.
About the authors
 We are both professional scientists, but only amateur Guinness drinkers. Although we had known for some time about the question of Guinness bubbles defying the norm, our curiousity was rekindled in 1998 with an article by Mark Buchanan for the December issue of The New Scientist (vol. 160, issue 2165, 19 December 1998). A few members of the Zare lab made a series of preliminary experiments at the local pub one Friday evening, however the results were not conclusive. We felt that the waves of dark liquid that flow down only gave the illusion that the bubbles were going down. The bubbles were just too small and too fast to see clearly, and by the time you started to form the impression that they were going down, the pint had settled and needed drinking!
We are both professional scientists, but only amateur Guinness drinkers. Although we had known for some time about the question of Guinness bubbles defying the norm, our curiousity was rekindled in 1998 with an article by Mark Buchanan for the December issue of The New Scientist (vol. 160, issue 2165, 19 December 1998). A few members of the Zare lab made a series of preliminary experiments at the local pub one Friday evening, however the results were not conclusive. We felt that the waves of dark liquid that flow down only gave the illusion that the bubbles were going down. The bubbles were just too small and too fast to see clearly, and by the time you started to form the impression that they were going down, the pint had settled and needed drinking!
In mid-2000 we tried some controlled experiments, using a regular digital video camera with a good zoom lens and a large magnifying desk lamp. Once again, it was difficult to say whether the waves of liquid were causing an optical illusion… or were those sneaky bubbles really going down?
In 2002 it was time to call in the heavy guns. We had heard a report that the sinking bubbles had been modelled using a commercial software package by Prof. Clive Fletcher at the University of New South Wales in Australia. Well, seeing is believing, we figured, so we set out to get the proof.
 Andy Alexander is a Royal Society University Research Fellow at the University of Edinburgh, Scotland. You can find out more about Dr Alexander’s work on the University of Edinburgh School of Chemistry webpage, or on his research group webpage. Andy Alexander is a Royal Society University Research Fellow at the University of Edinburgh, Scotland. You can find out more about Dr Alexander’s work on the University of Edinburgh School of Chemistry webpage, or on his research group webpage. |
 Dick Zare is the Marguerite Blake Wilbur Professor in Natural Science at Stanford University, CA, USA. You can find out more about Prof. Zare’s work on the Stanford University Chemistry Department webpage, or on his research group webpage. Dick Zare is the Marguerite Blake Wilbur Professor in Natural Science at Stanford University, CA, USA. You can find out more about Prof. Zare’s work on the Stanford University Chemistry Department webpage, or on his research group webpage. |
Frequently Asked Questions (FAQ)
Is the shape of the glass important?
The shape of the glass is not that important. We obtained similar results in curved and straight glasses. The pattern of flow will be different in the different shaped glasses, but not to the extent that would remove the effect.
Is the gas important?
Yes, it is.
Method of pouring?
The way the Guinness is poured is not crucial. Despite the angle/position of pouring, it is the upward flow of bubbles in the center of the glass that causes the circulation. If you pour it so that there are hardly any bubbles produced, then you will not see the effect.
What about viscosity?
More viscous liquids flow more slowly (e.g., syrup). The viscosity of Guinness will be only slightly different than that of water, but not much. Certainly not enough to affect the movement of the bubbles that can be seen.
Guinness in the lab?
You might wonder how we got a good supply of Guinness in the lab. The experiments took many days, and it would have been difficult to set up all the equipment in the local bar! Fortunately, the Guinness company makes their draught stout available in cans, using a special patented pouring device (the “widget”) that creates all those creamy bubbles. Although we tried refrigeration before using them, condensation on the glass caused difficulties, so we used the cans at room temperature.
What happened to the Guinness you used?
Alas, we poured it away. Don’t be sad – you might never have seen any of the pictures if we had actually drunk any of it during filming!
What’s a widget?
The widget is the patented device (also called a “smoothifier”) in the special draught-flow can or bottle that contains a small amount of Guinness and nitrogen gas at very high pressure. If you shake one of the cans you can hear it rattle. Why not open a can up and have a look? When the can/bottle is opened, the gas is suddenly released through one (or more) pin-sized holes. This creates a lot of little bubbles.
Do the simulations and experiments agree?
Our experiments and conclusions agree very well with the simulations carried out by Prof. Clive Fletcher and his group. The sinking bubbles in the experiments seem to be slightly larger than in the simulations. The experiments also suggest that the bubbles inside (that go up) seem to be nearer to the walls in the experiments. An interesting detailed comparison would come from looking just below the head, where we see almost equal numbers of bubbles going up and down.
What the response was in Australia
Here’s cheers for that scientific sinking feeling
By Richard Macey
March 20, 2004
This story was found at: http://www.smh.com.au/articles/2004/03/19/1079199418340.html
Copyright © 2004. The Sydney Morning Herald.

Bursting a scientific bubble Md Nurul Hasan Khan discovered that in a glass of Guinness bubbles sink, as well as rise – a theory now accepted by the science world. Photo: Glenn Hunt
If he was a drinking man, Md Nurul Hasan Khan would have popped into his local pub this week to celebrate by downing a cold beer.
But the chemical engineer and devout Muslim, who is now with the CSIRO’s minerals division, was simply chuffed to know the world finally accepted he was right. Bubbles in a glass of Guinness do sink, as well as rise.
In 1999 Mr Khan, known as Hasan by his friends, used mathematical computational modelling to explain the falling bubbles, something he had seen many times. “I don’t drink, but all my colleagues do, and I hang out with them.”
It didn’t make sense. Gas bubbles, lighter than beer, should only rise. Mr Khan, who did a PhD thesis on bubble behaviour in liquids, believed computational modelling held the answer.
He published his results, but the scientific world remained sceptical until this week when Scottish and Californian scientists announced slow-motion movies of Guinness confirmed its bubbles do fall.
Richard Zare, a professor at California’s Stanford University, said he had been aware of the Australian research: “I first disbelieved this and wondered if people had maybe too much Guinness to drink.”
To make his discovery, Mr Khan, then at the University of NSW’s school of chemical engineering and industrial chemistry, bought a Guinness glass and called the company for information about its viscosity, density and the typical size of its bubbles. He stirred the data in his computer, producing a mathematical model of the world inside every Guinness glass.
“I calculated all the forces acting on individual bubbles and I found some of the bubbles, near the sides of the glass, were going down.”
The model revealed that rising bubbles formed a column in the middle of the glass. “But those bubbles try to drag the liquid up,” he said. The rising beer spread out at the top of the glass, forcing beer at the sides to go down. Small bubbles on the sides were swept down with the flow. “It took me about six months of work, part time . . . now they [the Scottish and Californian scientists] have found the same thing. I am very pleased.”
The Bangladesh-born Mr Khan believes his discovery has significance for breweries and drinkers, because the longer bubbles circulate, the longer beer maintains its taste and smell. He now intends to find a way to keep the bubbles moving.
“Most people do not know computational modelling has some great value,” he said.
Riddle of beer bubbles solved in time for St. Patrick’s Day – Stanford Report 3/17/04
Stanford Report, March 17, 2004
Riddle of beer bubbles solved in time for St. Patrick’s Day
BY MARK SHWARTZ
A new experiment by chemists from Stanford University and the University of Edinburgh has finally proven what beer lovers have long suspected: When beer is poured into a glass, the bubbles sometimes go down instead of up.

Chemistry Professor Richard Zare hoists a pint of Guinness at the Empire Grill & Tap Room in Palo Alto after proving, with the help of former Stanford postdoctoral student Andrew Alexander, what beer lovers have long suspected: When beer is poured into a glass, the bubbles sometimes go down instead of up. Photo: L.A. Cicero
“Bubbles are lighter than beer, so they’re supposed to rise upward,” said Richard N. Zare, the Marguerite Blake Wilbur Professor in Natural Sciences at Stanford. “But countless drinkers have claimed that the bubbles actually go down the side of the glass. Could they be right, or would that defy the laws of physics?”
This frothy question reached a head in 1999 after Australian researchers announced that they had created a computer model showing that it was theoretically possible for beer bubbles to flow downward. The Australians based their simulation on the motion of bubbles in a glass of Guinness draught – a popular Irish brew that contains both nitrogen and carbon dioxide gas.
But Zare and former Stanford postdoctoral fellow Andrew J. Alexander were skeptical of the virtual Guinness model and decided to put it to the test by analyzing several liters of the liquid brew.
“Indeed, Andy and I first disbelieved this and wondered if the people had had maybe too much Guinness to drink,” Zare recalled. “We tried our own experiments, which were fun but inconclusive. So Andy got hold of a camera that takes 750 frames a second and recorded some rather gorgeous video clips of what was happening.”
Bottoms up, bubbles down
A careful analysis of the video confirmed the Australian team’s findings: Beer bubbles can and do sink to the bottom of a glass. Why does this happen?
“The answer turns out to be really very simple,” Zare explained. “It’s based on the idea of what goes up has to come down. In this case, the bubbles go up more easily in the center of the beer glass than on the sides because of drag from the walls. As they go up, they raise the beer, and the beer has to spill back, and it does. It runs down the sides of the glass carrying the bubbles — particularly little bubbles — with it, downward. After a while it stops, but it’s really quite dramatic and it’s easy to demonstrate.”
The phenomenon also occurred in other beers that did not contain nitrogen, said Alexander, now a professor at the University of Edinburgh in Scotland. “The bubbles are small enough to be pushed down by the liquid,” he said. “We’ve shown you can do this with any liquid, really — water with a fizzing tablet in it, for example.”
Confirmation of the sinking-bubble phenomenon has relevance beyond settling barroom bets, according to the researchers.
“There’s a certain aspect of bubbles that always make you think it’s kids’ play and relaxation, but it’s serious stuff, too,” Zare said, pointing to ongoing research on fluidized beds — the mixing of solid particles with liquids and gases — which have important industrial and engineering applications.
“It’s just paying attention to the world around you and trying to figure out why things happen the way they do,” Alexander added. “In that case, anyone that goes into a pub and orders a pint of Guinness is a scientist.”
Media Coverage
Stanford News Service video interview
St. Patty’s Broadcast on ABC Channel 7 news- Interview with Dr. Zare 3/17/04
First public announcement of understanding the settling of Guinness
