Microdroplet Reactions
Xin Yan, Yin-hung Lai
A recent finding worthy of attention is that many reactions can be dramatically accelerated in microdroplets compared to the traditional bulk synthesis, with acceleration factors measured by on-line mass spectrometry from 2 to 6 orders of magnitude.1,2 The chemistry represented in single phase reactions includes carbon–carbon bond formation, carbon–nitrogen bond formation, cyclization, oxidation–reduction, and multicomponent multi-step reactions. Many factors are thought to contribute to the reaction acceleration such as microdroplet evaporation, confinement of reagents, alteration of pH of the microdroplet surface, and probably one of the most important features, the high surface-to-volume ratio of the microdroplet. In fact, microdroplet reactors provide an efficient platform to perform superfast two-phase reactions without using a phase-transfer catalyst (Figure 1).
Many important chemical transformations occur in two-phase reactions and are widely used in chemical, pharmaceutical, and polymer manufacturing. We present an efficient methodology to perform two-phase reactions in microdroplets sheared by sheath gas without using a phase-transfer catalyst.3 This avoids disadvantages such as thermal instability, cost, and especially separation and recycling of the catalysts. We show that various alcohols can be oxidized to the corresponding aldehydes and ketones within milliseconds in moderate to good yields (50-75%). The scale-up of the present method was achieved at a rate of 1.6 mg/min for the synthesis of 4-nitrobenzylaldehyde from 4-nitrobenzyl alcohol in the presence of sodium hypochlorite. The biphasic nature of this process that avoids use of a phase transfer catalyst greatly enhances synthetic effectiveness.
Oxidations of aldehydes to carboxylic acids are fundamentally important chemical transformations that have long interested academia and industry. We report a highly efficient and selective aerobic oxidation of aldehydes to carboxylic acids in microdroplets. Molecular oxygen plays two roles: (1) as the nebulizing gas to shear the aldehyde solution into microdroplets, and (2) as the sole oxidant. The dramatic increase of the surface area-to-volume ratio of microdroplets compared to bulk solution, and the efficient mixing of gas and liquid phases using spray nozzles allow effective mass transfer between aldehydes and molecular oxygen. The addition of catalytic nickel(II) acetate can further accelerate microdroplet reactions. We show that aliphatic, aromatic, and heterocyclic aldehydes can be oxidized to the corresponding carboxylic acids in a mixture of water and ethanol, in moderate to excellent yields (62–91%). We also scaled up the microdroplet synthesis to make it preparative. Aerobic oxidation of 4-tert-butylbenzaldehyde to 4-tert-butylbenzoic acid was achieved at a rate of 10.5 mg/min for the isolated product at a yield of 66%.
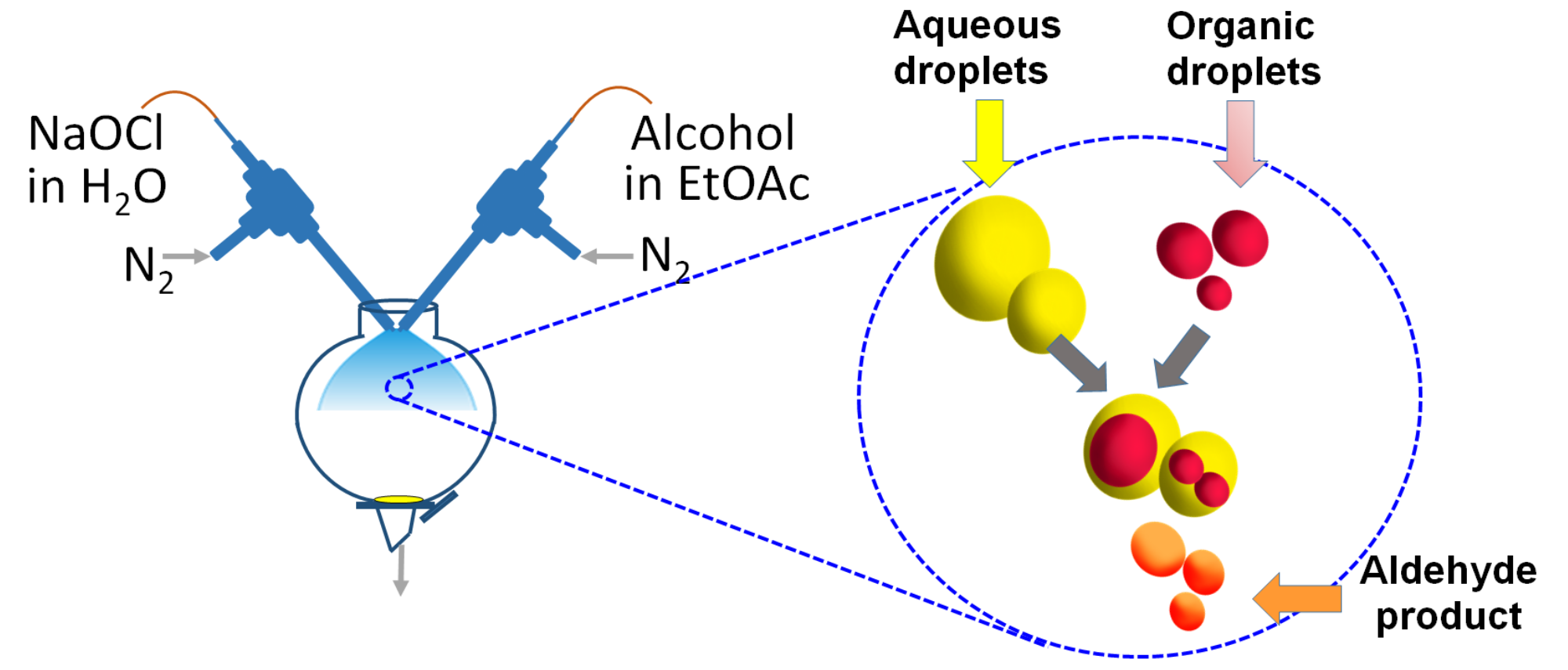
Figure 1. Biphasic reactions are carried out in microdroplets without using a phase-transfer catalyst. Reactions occur within milliseconds in moderate to good yields.
1. Yan X, Bain RM, Cooks RG. 2016. Organic Reactions in Microdroplets: Reaction Acceleration Revealed by Mass Spectrometry. Angewandte Chemie International Edition 55: 12960-72 2. Banerjee S, Gnanamani E, Yan X, Zare RN. 2017. Can all bulk-phase reactions be accelerated in microdroplets? Analyst 142: 1399-402 3. Yan X, Cheng H, Zare RN. 2017. Two-Phase Reactions in Microdroplets without the Use of Phase-Transfer Catalysts. Angewandte Chemie International Edition 56: 3562-65
